Vivasc Therapeutics is built upon the proprietary Cardiac Targeting Peptide (CTP) platform technology.
CTP is a synthetic, non-naturally occurring peptide that acts as a novel vector to target the heart and has demonstrated ability to transduce:
- Normal mouse hearts in vivo (peak uptake at 15 minutes after injection)
- Explanted human heart tissue
- Human derived iPSC beating cardiomyocytes
CTP demonstrates robust transduction of normal cardiomyocytes and sparing of fibroblasts present in adjacent scar tissue. To date, CTP conjugates have transduced cardiomyocytes carrying intact cargo as diverse as nucleic acids, radioisotopes, therapeutics, and other peptides.
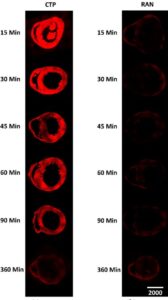
The above image is fluorescence microscopy of murine hearts injected with cardiac targeting peptide-cyanine 5.5-N-Hydroxysuccinimide (CTP) vs. a random peptide (RAN) at 10 mg/kg and euthanized at indicated time points.
Peak CTP uptake by heart tissue is seen at 15 min with a steady decline over time.
Source: Zahid, M., Feldman, K. S., Garcia-Borrero, G., Feinstein, T. N., Pogodzinski, N., Xu, X., Yurko, R., Czachowski, M., Wu, Y. L., Mason, N. S., & Lo, C. W. (2018). Cardiac Targeting Peptide, a Novel Cardiac Vector: Studies in Bio-Distribution, Imaging Application, and Mechanism of Transduction. Biomolecules, 8(4), 147. https://doi.org/10.3390/biom8040147
Cardiac Targeting Peptide (CTP) Publications
Title of Paper (Year)
Abstract
Targeted delivery of therapeutics specifically to cardiomyocytes would open up new frontiers for common conditions like heart failure. Our studies with CTP showed no activation or inhibition of GPCR-associated receptors in vitro. We found no signals indicative of toxicity in vivo. Most importantly, cardiac functions remained unchanged acutely in response to CTP uptake.
Despite significant strides in prevention, diagnosis, and treatment, cardiovascular diseases remain the number one cause of mortality in the United States, with rates climbing at an alarming rate in the developing world. The goal of this publication is to provide a comprehensive review of the identification, validation, and application of CTP, and outline its potential in diagnostic and therapeutic applications especially in the field of targeted RNA interference.
As one of the most efficient drugs known for the treatment of cardiac arrhythmia, Amiodarone presents the same conundrum of serious systemic side effects associated with long term treatment. Our in vivo studies in guinea pigs indicate that cardiac targeting peptide-amiodarone conjugate is able to have similar effects on calcium handling as amiodarone at 1/15th the total molar dose of amiodarone.
Amiodarone is underutilized due to significant off-target toxicities. We hypothesized that targeted delivery to the heart would lead to lowering of dose by utilizing a cardiomyocyte targeting peptide (CTP), a cell penetrating peptide identified by our prior phage display work.
We show that miRNA106a targets genes that, when misregulated, have been shown to cause hypertrophy and eventual Heart Failure (HF). The addition of miRNA106a suppresses misexpressed HF genes and reverses hypertrophy. Most importantly, using a cardiac targeting peptide reversibly linked to miRNA106a, we show delivery is specific to cardiomyocytes.
In this study, we evaluated the therapeutic potential of CTP-expressing sEVs (C-sEV) for the delivery of RAGE-targeting small interfering RNA (siRNA) (siRAGE) to suppress myocarditis. siRAGE-loaded C-sEVs attenuated inflammation in both cell culture and an in vivo model of myocarditis. Taken together, C-sEVs may be a useful drug delivery vehicle for the treatment of heart disease.
Curcumin exerts therapeutic effects in heart disease, but has limited bioavailability. Extracellular vesicles (EVs) have gained attention as nanovehicles; however, the poor targeting ability of systemically administered EVs still remains a crucial issue. Herein, we generated heart-targeted EVs (CTP-EVs) by functionalizing EVs surface with cardiac targeting peptide (CTP) using genetic modification of EVs-secreting cells, and further loaded curcumin into CTP-EVs (CTP-EVs-Cur). Consequently, CTP-EVs were able to specifically deliver curcumin to the heart. In addition, curcumin-loaded CTP-EVs possess improved bioavailability, and are fully functional with a high cardioprotective efficiency.
In this chapter, we use CTP as an example to describe techniques for validation of cell-specific transduction as well as provide details on a technology to identify binding partner(s) for these ever-increasing plethora of tissue-specific peptides. Given the myriad cargoes CTP can deliver, as well as rapid uptake after an intravenous injection, it can be applied to deliver radioisotopes, miRNA, siRNA, peptides, and proteins of therapeutic potential for acute cardiac conditions like myocardial infarction, where the window of opportunity for salvaging at-risk myocardium is limited to 6 hrs.
Since their initial description and characterization, the field of cell penetrating peptides as vectors has exploded. The cargoes they can deliver range from other small peptides, full-length proteins, nucleic acids including RNA and DNA, liposomes, nanoparticles, and viral particles as well as radioisotopes and other fluorescent probes for imaging purposes.
Our previous work identified a 12-amino acid peptide that targets the heart, termed cardiac targeting peptide (CTP). We now quantitatively assess the bio-distribution of CTP, show a clinical application with the imaging of the murine heart, and study its mechanisms of transduction.
This study evaluated whether genetic modification of exosomes could enhance exosome delivery to heart cells and heart tissue without toxicity. Exosomes expressing cardiac-targeting peptide (CTP)-Lamp2b on the exosomal membrane (CTP-Exo) were generated by introducing vectors encoding CTP-Lamp2b into HEK 293 cells. These results suggested that CTP-Exo might be used as a therapeutic tool for heart disease.
Despite recent improvements in techniques, ablation procedures are still limited by the risk of complications from unwanted cellular damage, caused by the nonspecific delivery of ablative energy to all heart cell types. We describe an engineered nanoparticle containing a cardiac-targeting peptide (CTP) and a photosensitizer, chlorin e6 (Ce6), for specific delivery to myocytes.
Here we review the potential applications as well as hurdles to the tremendous potential of Cell Penetrating Peptides, in particular the cell-type specific peptides.
The development of cell permeable (or penetrating) peptide tagged proteins has facilitated the delivery of Cre recombinase protein into cells in culture, organotypic slide culture, or in living animals. In this report, we generated bacterially expressed, his-tagged Cre protein with either a cardiac targeting peptide or an antennapedia peptide at the C-terminus and demonstrated efficient uptake and recombination in both cell culture and mice.
Targeting stem cells holds great potential for studying the embryonic stem cell and development of stem cell-based regenerative medicine. Previous studies demonstrated that nanoparticles can serve as a robust platform for gene delivery, non-invasive cell imaging, and manipulation of stem cell differentiation. However specific targeting of embryonic stem cells by peptide-linked nanoparticles has not been reported.
A peptide able to transduce cardiac tissue specifically, delivering cargoes to the heart, would be of significant therapeutic potential for delivery of small molecules, proteins and nucleic acids. In order to identify peptide(s) able to transduce heart tissue, biopanning was performed in cell culture and in vivo with a M13 phage peptide display library.